A recent groundbreaking discovery on Titan, Saturn’s largest moon, is poised to fundamentally redefine our understanding of its chemical processes. Researchers have now observed that frozen crystals of hydrogen cyanide possess the surprising ability to mix with liquid hydrocarbons – a combination previously thought to be chemically impossible. This unexpected revelation necessitates a comprehensive re-evaluation of the established chemical models governing this intriguing celestial body.
New research combining experiments at NASA’s Jet Propulsion Laboratory (JPL) in Southern California with computer simulations from Sweden’s Chalmers University of Technology has illuminated a unique chemical process on Saturn’s moon Titan.
Scientists have demonstrated how the liquid ethane and methane, abundant in Titan’s seas and lakes, can intermix with crystals of frozen hydrogen cyanide. This interaction occurs within the moon’s extraordinarily frigid environment, where temperatures plummet to a chilling minus 179 degrees Celsius.
Hydrogen cyanide is identified as a polar molecule, meaning it features a distinct separation of electric charge, with one end carrying a positive charge and the other a negative charge. This inherent polarity drives its behavior, making it predisposed to form bonds with other polar molecules, as the fundamental principle of opposite charges attracting comes into play.

Methane and ethane, both hydrocarbon compounds formed from hydrogen and carbon atoms, are distinctly non-polar molecules. This means their electric charge is symmetrically distributed, ensuring an even balance of positive and negative charges across their entire molecular structure.
A fundamental principle of chemistry dictates that substances with differing polarities—polar and non-polar compounds—typically do not intermix. This phenomenon is perhaps most famously illustrated by the natural separation of oil and water, where distinct layers persist rather than a unified solution forming.
Hydrogen cyanide (HCN) comes into existence within Titan’s atmosphere through the action of ultraviolet light, which dismantles existing hydrocarbons and reassembles them into new molecules. Given the abundant presence of non-polar hydrocarbons across Titan’s atmosphere and surface, researchers at NASA’s Jet Propulsion Laboratory (JPL) sought to understand the subsequent fate of this compound after its formation.
However, laboratory experiments simulating Titan’s frigid conditions – combining hydrogen cyanide with methane and ethane at a chilling minus 292 degrees Fahrenheit (minus 180 degrees Celsius) – yielded perplexing and inexplicable outcomes. Faced with these bewildering results, JPL turned to chemist Martin Rahm and his team at Chalmers University, renowned for their specialized expertise in hydrogen cyanide’s behavior at cryogenic temperatures, to assist in unraveling the mystery.
This development, Rahm stated, catalyzed an exciting theoretical and experimental collaboration between Chalmers and NASA. Central to their inquiry was a bold hypothesis: could existing measurements be explained by a unique crystal structure that integrates methane or ethane with hydrogen cyanide? This unconventional proposal, Rahm highlighted, directly challenges the fundamental chemical rule, ‘like dissolves like,’ which posits that polar and non-polar substances are inherently incompatible and should not combine.
Computer simulations conducted by Rahm have revealed a significant discovery: methane and ethane molecules possess the ability to permeate the crystal lattice of frozen hydrogen cyanide. This unique interaction leads to the formation of a novel and stable composite structure, which researchers have termed a “co-crystal.”
Rahm revealed that this phenomenon is not only possible but stable at the extreme, frigid temperatures found on Saturn’s moon, Titan. Their team’s advanced computational models predicted the surprising stability of specific chemical mixtures in Titan’s harsh environment. Crucially, these same models also generated light spectra that showed a significant and compelling correlation with data previously collected by NASA.
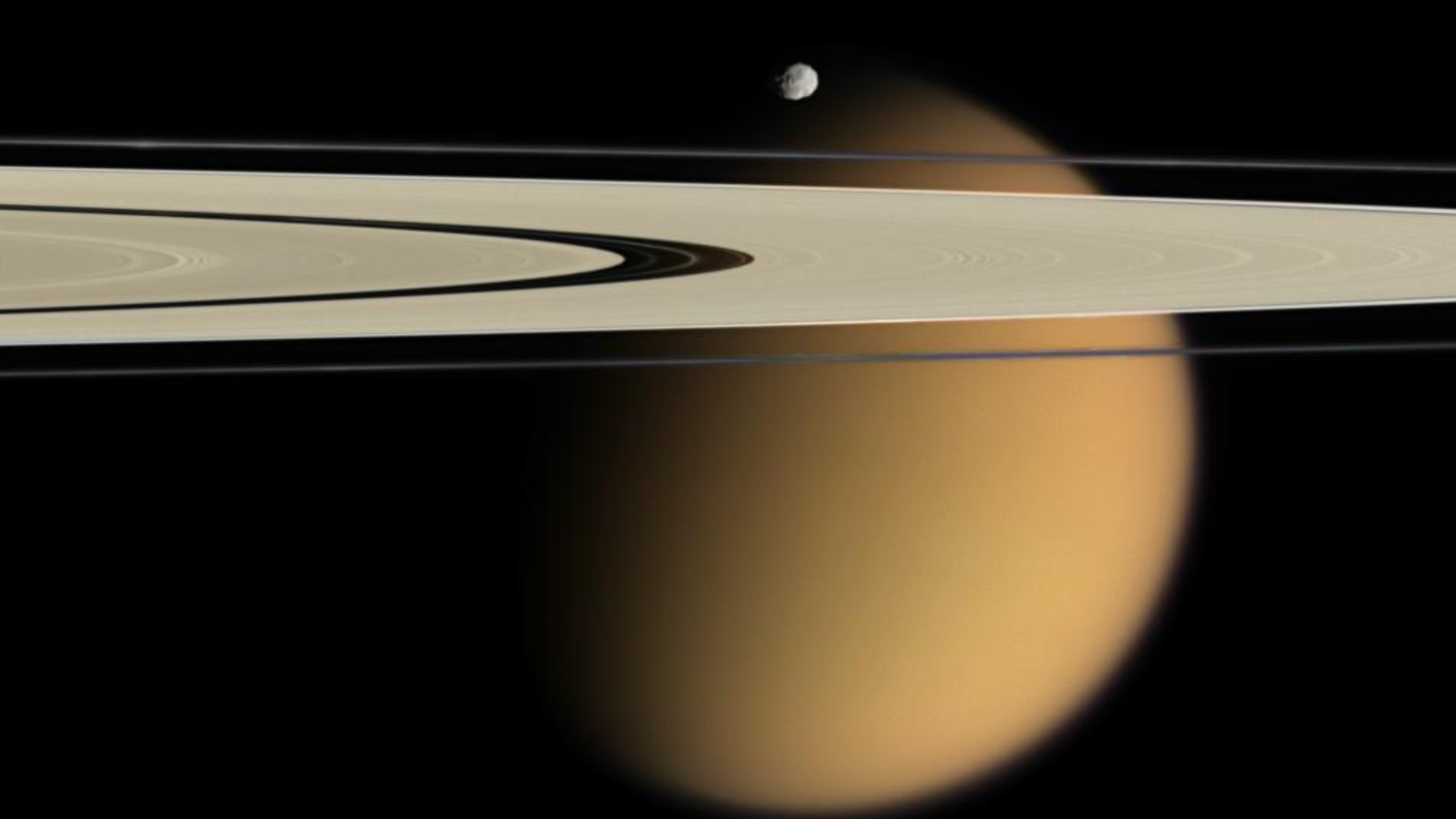
Saturn’s moon Titan stands as a unique celestial body, distinguished as the only moon in our solar system to possess a thick atmosphere. This dense, hydrocarbon-rich envelope bears a striking chemical resemblance to the “prebiotic soup” scientists hypothesize existed on Earth before life began.
While Titan’s frigid temperatures generally preclude the kinds of chemical reactions necessary for life as we currently understand it, its profound astrobiological significance lies in its ability to serve as a crucial analogue. It offers a valuable snapshot of the molecular inventory that may have characterized early Earth. A prime example is hydrogen cyanide (HCN). Despite its toxicity to modern life, HCN is recognized as a fundamental building block for vital biological compounds, including the amino acids that form proteins and the nucleobases essential for RNA and DNA.
Hydrogen cyanide (HCN) is a remarkably prevalent molecule throughout the cosmos, detected in expansive dust clouds, planetary atmospheres, and comets, noted scientist Rahm. The insights gleaned from this study are expected to deepen our understanding of chemical reactions occurring in other extremely cold environments across space. Moreover, the research paves the way for investigations into whether other non-polar molecules might similarly incorporate into hydrogen cyanide crystals and, crucially, what implications such interactions could hold for the chemistry that predates the emergence of life.
Recent scientific findings reveal a far more intricate and dynamic interplay among Titan’s atmosphere, its expansive frozen ice dunes, and its liquid methane and ethane lakes and seas than previously understood. This enhanced understanding will be put to the test by NASA’s Dragonfly mission.
Scheduled to arrive at Titan in 2034, the innovative rotorcraft will conduct a series of surface stops. During these explorations, Dragonfly will meticulously collect samples, including hydrogen cyanide ice. This material is crucial for verifying the new results and searching for even more complex and unexpected chemical processes occurring on the distant moon.
Here are a few options, maintaining the core meaning with a journalistic tone:
* The research findings were published in July in the scientific journal PNAS.
* The journal PNAS unveiled the findings in its July publication.
* Details of the discoveries appeared in July’s edition of PNAS.
* The study’s conclusions were detailed in July within the pages of PNAS.